Key points:
- Marker Therapeutics (MRKR) stock soared 126.52% on FDA approval.
- The company will begin phase 1 studies of its MT-601 cancer drug.
- The drug is unique in the market as it targets untreatable patients.
The Marker Therapeutics Inc (NASDAQ: MRKR) stock price soared 126.52% premarket after announcing that the US Food and Drug Administration (FDA) has approved its Investigational New Drug (IND) application for MT-601, a multi-tumour-associated antigen (multiTAA)-specific T cell product targeting six antigens.
The drug candidate is meant to treat patients suffering from relapsed/refractory non-Hodgkin lymphoma who are ineligible for anti-CD19 CAR T cell treatment. Investors cheered the FDA clearance allowing Marker to start phase I trials.
Also read: The Best Biotech Penny Stocks Under $5 To Buy Right Now.
Maker intends to focus on r/r NHL patients who have failed CD19 CAR-T therapy and those who do not qualify for treatment using such therapies. In addition, the drug brings hope to cancer patients whose tumours have escaped by downregulating the expression of CD19.
The company will use high doses of 200 million cells per infusion since the MT-601 does not have the adverse side effects associated with other cell therapies like TCRs and CAR-Ts.
Dr Mythili Koneru, Marker’s Chief Medical Officer, said: “This new clinical trial will build upon results that were observed in Phase I/II TACTAL study conducted by BCM, which assessed the safety and efficacy of five-antigen-directed multiTAA-specific T cell product,”
Adding:
“In the TACTAL study, BCM observed long-term CR rates that were comparable to recently approved CD19 CAR-T therapies, even at very low cell doses. Unlike CD19 CAR-T cell therapies, patients receiving multiTAA-specific T cell product had superior durability of response, without the severe toxicities that commonly occur with other adoptive cell therapies, such as cytokine release syndrome or neurotoxicity. Based on these results, we believe that multiTAA-specific T cell products can be easily administered in an outpatient setting without hospitalization.”
Peter L. Hoang, Marker’s President and Chief Executive Officer, added: “FDA clearance of our IND for MT-601 is a significant milestone as we advance our pipeline in a number of Company-sponsored trials. We believe that MT-601, which targets six tumor-associated antigens highly expressed in lymphoma, has the potential to build upon the results of the TACTAL study. We look forward to initiating our Company-sponsored Phase 1 study next year.”
*This is not investment advice. Always do your due diligence before making investment decisions.
Marker Therapeutics stock price.
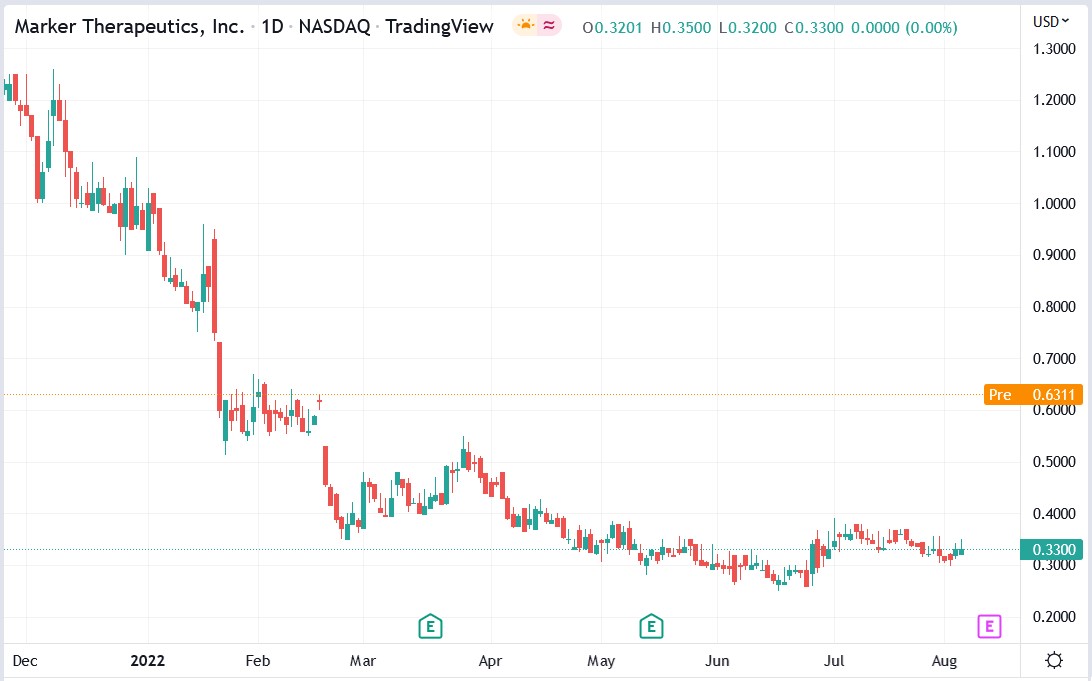
Marker Therapeutics stock price soared 126.52% to trade at $0.7475, rising from Thursday’s closing price of $0.3300.
