The Immuron ADR (NASDAQ: IMRN) stock price surged 58.3% after the US Food and Drug Administration (FDA) lifted its clinical hold on the company’s New Campylobacter ETEC Therapeutic IND application.
YOUR CAPITAL IS AT RISK. 76% OF RETAIL CFD ACCOUNTS LOSE MONEY.
The Australian-based global biopharma company noted that the US Naval Medical Research Centre (NMRC) had satisfactorily addressed all the clinical hold issues identified by the FDA, leading to its lifting.
Top Broker Recommendation
- eToro Top stock trading platform with 0% commission – Read our Review
- Tickmill Regulated by the FCA – Read our Review
- Admirals (Admiral Markets) More than 4500 stocks & ETFs available – Read our Review
- Spreadex Spreadex has been around for a long time – Read our Review
- IG Top-tier regulation – Read our Review
YOUR CAPITAL IS AT RISK. 76% OF RETAIL CFD ACCOUNTS LOSE MONEY
The company has developed two commercially-available oral immunotherapeutic products that treat gut-mediated diseases.
In addition, Immuron confirmed that the US Naval Medical Research Center (NMRC) had received approval from the FDA to proceed with the clinical evaluation of the oral therapy targeting Campylobacter and Enterotoxigenic Escherichia coli (ETEC).
The US NMRC developed the drug in partnership with Immuron and is responsible for most clinical development work, including the IND application. The FDA’s lifting of the clinical hold will allow the US NMRC to continue with its plans to evaluate the efficacy of the hyperimmune drug in preventing infectious diarrhoea caused by Campylobacter and ETEC.
The drug will now enter two clinical trials testing its safety and protective efficacy. One of the clinical trials will focus on the ability of the hyperimmune product to protect volunteers against ETEC infections. In contrast, the second trial will focus on moderate to severe campylobacteriosis.
The two trials will involve 60 volunteers who will be split randomly into two groups of 30 each, with Cohort 1 being the ETEC group and Cohort 2 being the one exposed to C. jejuni.
The first clinical study will be at the Johns Hopkins University (JHU) Center for Immunization Research (CIR) Inpatient Unit at the Johns Hopkins Bayview Medical Campus. The study will include 30 healthy participants aged 18-50 years.
The rationale for the clinical trial and the drug is based on the fact that infectious diarrhoea is the most common illness reported by travellers visiting developing countries and among US troops deployed overseas.
The morbidity and associated discomfort from diarrhoea decreases daily performance, lowers morale, affects judgment and reduces operational readiness. The first treatment for infectious diarrhoea is antibiotics, which are no longer very effective.
Hence, a preventative treatment that protects against enteric diseases is a high-priority objective for the US Military.
*This is not investment advice.
Immuron stock price.
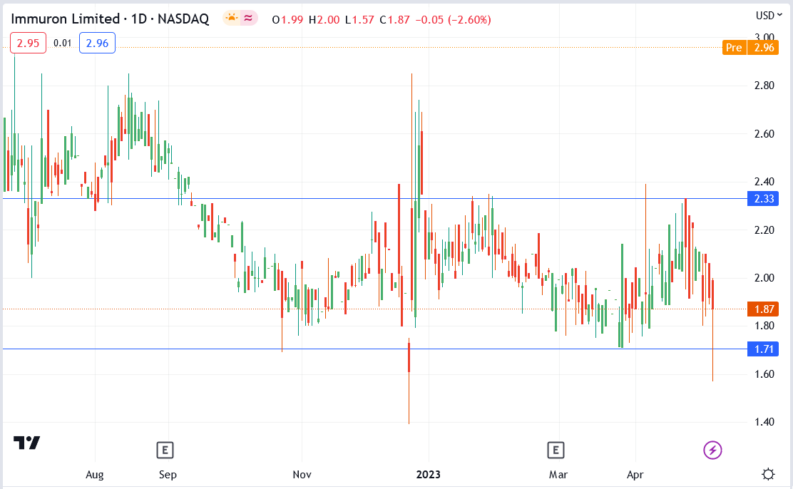
The Immuron stock price surged 58.29% to $2.96, from Friday’s closing price of $1.87.
YOUR CAPITAL IS AT RISK. 76% OF RETAIL CFD ACCOUNTS LOSE MONEY.









