The ImmuPharma PLC (LON: IMM) share price fell 18.4% after releasing its final results for the year ended 31 December 2022. The company lost £3.8 million, marking a significant improvement to the £8.2 million loss it incurred in the previous year.
YOUR CAPITAL IS AT RISK. 76% OF RETAIL CFD ACCOUNTS LOSE MONEY.
The company’s research and development spending fell to £2.0 million from £3.7 million in 2021, while its administrative expenses dropped to £0.8 million from £1.0 million in 2021. As a result, the company had zero exceptional items in 2022 compared to exceptional items worth £1.4 million from corporate reorganisation costs in 2021.
Top Broker Recommendation
- eToro Top stock trading platform with 0% commission – Read our Review
- Tickmill Regulated by the FCA – Read our Review
- Admirals (Admiral Markets) More than 4500 stocks & ETFs available – Read our Review
- Spreadex Spreadex has been around for a long time – Read our Review
- IG Top-tier regulation – Read our Review
YOUR CAPITAL IS AT RISK. 76% OF RETAIL CFD ACCOUNTS LOSE MONEY
ImmuPharma had a cash balance of £0.7 million as of 31 December 2022, compared to £1.6 million in December 2021, despite raising £2.0 million via a share subscription in August 2022. The company also had other financial assets worth over £1.0 million.
Other operational milestones achieved by the company include P140 Pharmacokinetic (PK) study completed in April 2022, with critical findings shared with the FDA. In addition, in February 2023, an adaptive Phase 2/3 study for Lupuzor™ in SLE/Lupus patients was agreed upon with US partner Avion Pharmaceuticals, following guidance from the FDA.
ImmuPharma has also confirmed a Type C meeting with the FDA for 7 June 2023, where the FDA will consider its new protocol of the Phase 2/3 adaptive study for Lupuzor™ in SLE patients.
There is also a Pre-IND meeting confirmed with the FDA for 16 May 2023, where the FDA will hive feedback on the new protocol for the Phase 2/3 adaptive clinical trial for CIPD.
Tim McCarthy, ImmuPharma’s CEO, commented: “As a Board, we remain focused on bringing our two key late-stage clinical assets, Lupuzor™ for lupus and CIDP, closer to the market. We now have a clinical roadmap for Lupuzor™ and remain on track to commence the Phase 2/3 adaptive trial in H2 2023, with potential CIDP moving into clinical studies in parallel. This illustrates the potential franchise we have within our P140 autoimmune platform.”
The CEO added that the firm would remain focused on commercial and partnering opportunities while closely monitoring its cost base.
*This is not investment advice.
ImmuPharma (IMM) share price.
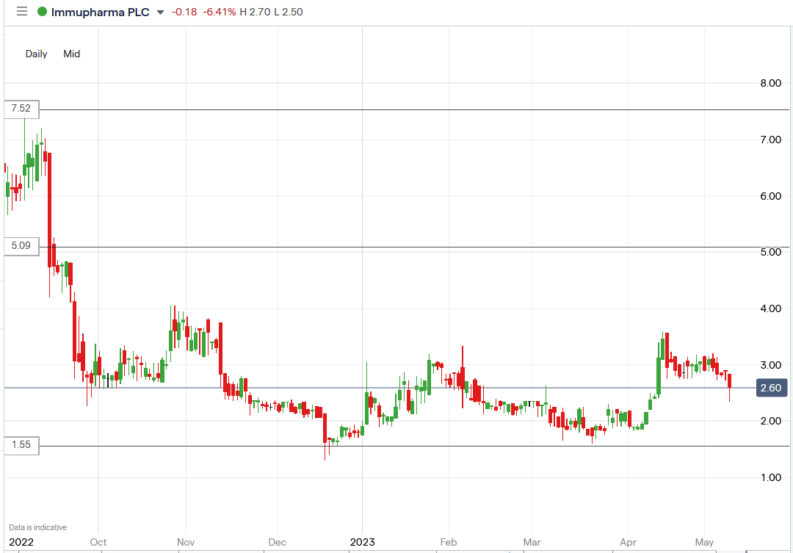
The ImmuPharma share price fell 18.4% to trade at 2.35p, from Wednesday’s closing price of 2.88p.
YOUR CAPITAL IS AT RISK. 76% OF RETAIL CFD ACCOUNTS LOSE MONEY.









