
Shares in biotechnology company AnPac Bio (NASDAQ:ANPC) have surged after the company announced that it has been validating the approved COVID-19 antibody test, for commercial use in its San Jose lab…
The company said that the validation is expected to be completed in the second half of 2020.
With COVID-19 cases still on the rise, especially in the US, AnPac feels as though there will be long term and widespread demand for the antibody test…
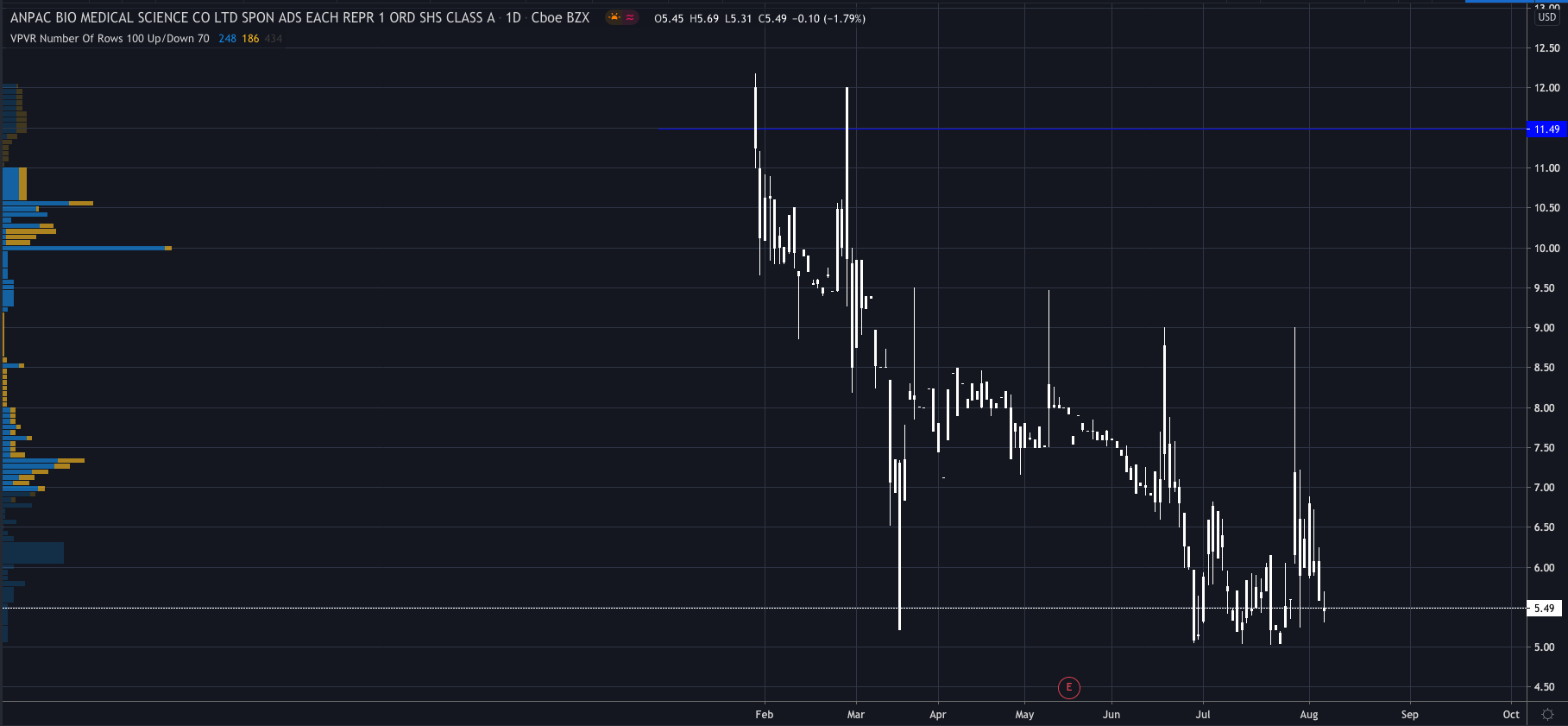
And investors seem to think so too as its shares surged over 100% higher after the announcement and are trading at $11.49 premarket at 14:02 BST, its highest level since late February.
AnPac Bio’s CEO, Dr. Chris Yu, commented, “Following the successful launch of our immunology product and CDA/ct-DNA combination test product in the first half of this year, the pending commercialization of the approved COVID-19 antibody test will be another major new product and service which AnPac Bio will offer that will accelerate our revenue growth.
“Our CLIA and CAP accredited clinical laboratory in the US allows us to take advantage of commercial market opportunities in the US by offering additional laboratory services while also continuing our research and development on our CDA technology.”
