The OKYO Pharma Ltd (LON: OKYO) share price rallied 14.12% after announcing that the first patient in its phase 2 clinical trial of its OK-101 drug candidate for treating dry eye disease by addressing a significant unmet need in the multi-billion dollar industry.
YOUR CAPITAL IS AT RISK. 76% OF RETAIL CFD ACCOUNTS LOSE MONEY.
The company revealed that the first patient had been screened for its phase 2, multi-centre, randomized, double-blinded, placebo-controlled trial. The trial’s ultimate goal is to evaluate the efficacy and safety of OK-101 as an ophthalmic solution in patients with dry eye disease (DED).
Top Broker Recommendation
- eToro Top stock trading platform with 0% commission – Read our Review
- Admiral Markets More than 4500 stocks & ETFs available – Read our Review
- BlackBull 26,000+ Shares, Options, ETFs, Bonds, and other underlying assets – Read our Review
- IG Top-tier regulation – Read our Review
- XTB UK regulated by the FCA – Read our Review
YOUR CAPITAL IS AT RISK. 76% OF RETAIL CFD ACCOUNTS LOSE MONEY
OKYO Pharma expects to release the topline data from the clinical trial by the end of 2023. If successful, the study would act as one of two phase 3 clinical studies mandated by the US Food and Drug Administration (FDA) for approval.
The company also noted that dry eye disease affects over 49 million people in the United States alone and is hard to diagnose and treat due to the multifactorial nature of the condition.
Gary S. Jacob, PhD, OKYO Pharma’s CEO, said: “The initiation of this trial of topically applied OK-101 to treat dry eye disease marks a significant step for the company as we have been laser-focused on moving this drug candidate into clinical trials over the last 18 months. Importantly, this first clinical study is designed to include pre-specified primary efficacy endpoints, which are the hallmark of phase 3 registration trials and the results from this trial are anticipated before the end of this year.”
Gabriele Cerrone, OKYO Pharma’s Executive Chairman and Founder, said: “One of the most exciting aspects of this innovative clinical program is that we can get a rapid and informative answer on both safety and efficacy of OK-101 by the end of the year. Furthermore, positive results would allow us to expedite the program towards FDA approval by leveraging results from this phase 2 dry eye trial in lieu of one of the two required phase 3 trials needed to support U.S. marketing authorization. OKYO remains well-positioned as novel ophthalmic compounds in large markets represent promising acquisition targets as evidenced by the recent $5.9 billion Iveric deal.”
*This is not investment advice.
The OKYO Pharma share price.
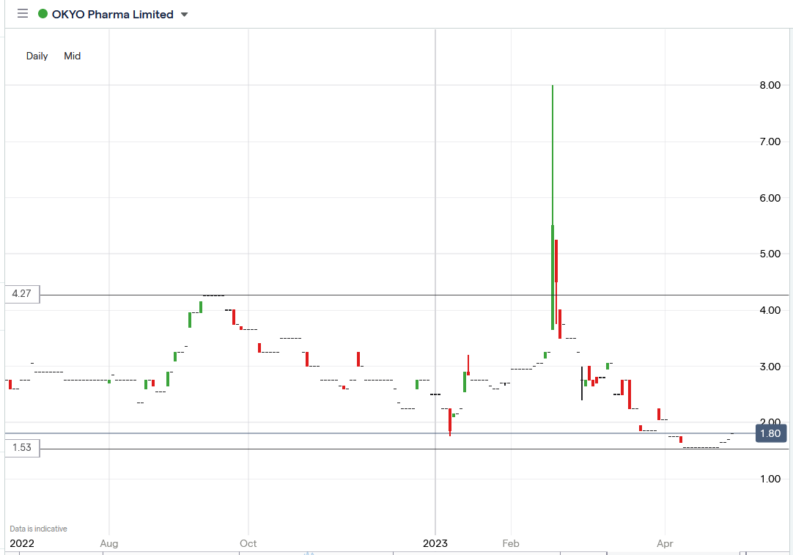
The OKYO Pharma share price surged 14.12% to trade at 1.94p, from Friday’s closing price of 1.70p.
YOUR CAPITAL IS AT RISK. 76% OF RETAIL CFD ACCOUNTS LOSE MONEY.










