
Cassava Sciences (NASDAQ: SAVA) shares have rocketed in Tuesday trading after it said interim analysis from an open-label study of simufilam, its lead drug candidate for treating Alzheimer’s disease, showed an improvement in patients’ cognition and behaviour following six months of treatment.
Cognition scores improved by 1.6 points on ADAS-Cog11, a 10% mean improvement from baseline to month six.
Simufilam also improved dementia-related behaviour, such as anxiety, delusions and agitation by 1.3 points on the Neuropsychiatric Inventory, a 29% mean improvement from baseline to month six.
“We could not be more pleased with these interim results,” said Remi Barbier, President & CEO.
“We would have been satisfied to show simufilam stabilises cognition in patients over 6 months. An improvement in cognition and behavior tells us this drug candidate has potential to provide lasting treatment effects for people living with Alzheimer’s disease. It’s an exciting development,” Barbier added.
The company also said there were no safety issues with no drug-related serious adverse events.
Today’s results have supported Cassava’s case for advancing simufilam to a Phase 3 clinical program in Alzheimer’s disease, and the company remains on track to initiate Phase 3 trials in the second half of 2021.
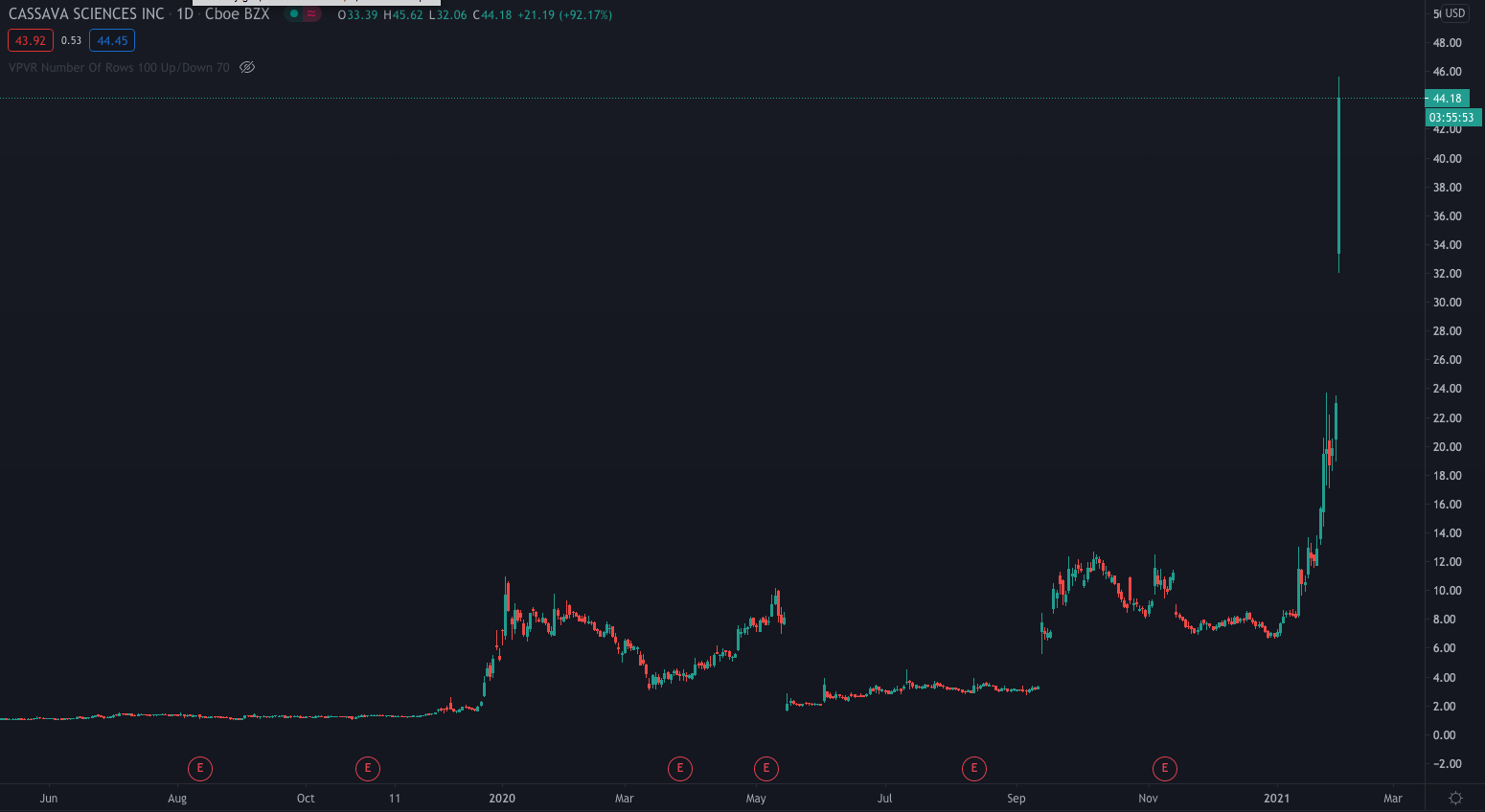
It is no surprise Cassava's shares have rocketed over 93% to $44.45 following the announcement. As it's President stated, stabilisation in cognition and behaviour would have been a positive step, so the improvement is a massive bonus and provides an exciting platform for the company in developing the treatment.
