
Novavax (NASDAQ: NVAX) stock has climbed over 6% after it announced that it has started its Phase 3 experimental COVID-19 vaccine trial in partnership with the UK’s Vaccines Taskforce.
The company said the trial is expected to enrol up to 10,000 individuals over the next four to six weeks, between the ages of 18-84 with and without pre-existing conditions.
“With a high level of SARS-CoV-2 transmission observed and expected to continue in the UK, we are optimistic that this pivotal Phase 3 clinical trial will enroll quickly and provide a near-term view of NVX-CoV2373’s efficacy,” said Gregory M. Glenn, M.D., President, Research and Development at Novavax.
The company said that the data from the trial will support regulatory submissions for licenses in the UK, EU and other countries.
25% of patients enrolled in the trial will be over the age of 65, while 400 of the patients will also receive the flu vaccine as part of the study.
In its press release, Novavax said the trial has two primary endpoints. Firstly that there is the “first occurrence of PCR-confirmed symptomatic COVID-19 with onset at least 7 days after the second study vaccination in volunteers who have not been previously infected with SARS-CoV-2.”
The second is “first occurrence of PCR-confirmed symptomatic moderate or severe COVID-19 with onset at least 7 days after the second study vaccination in volunteers who have not been previously infected with SARS-CoV-2.”
Novavax shares…
Novavax’s stock surged in after-hours trading after the announcement. It is currently up 6.75% at $109.35 premarket on Friday.
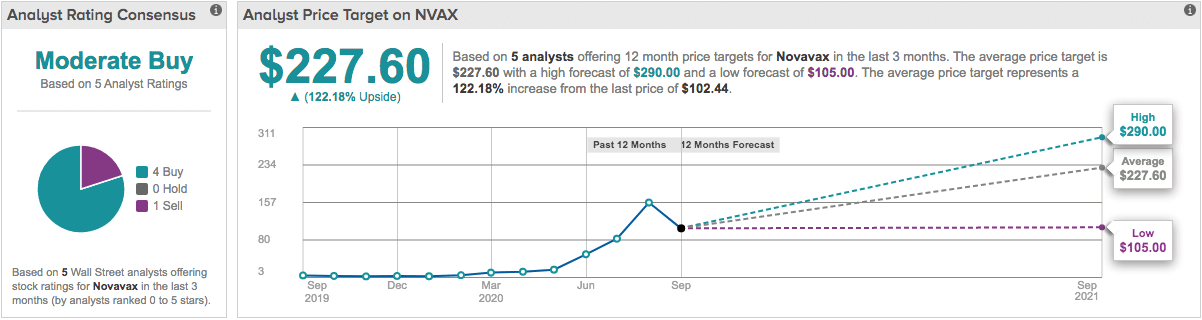
Analysts surveyed by TipRanks show a median 12-month price forecast of $227.60 for Novavax shares representing a potential 122% upside for the stock from its close on Thursday. Four of the five analysts rated the stock as a buy with one rating it as a sell.
PEOPLE WHO READ THIS ALSO VIEWED:
- TAT Technologies stock rallies after Honeywell agreement
- Trade stocks with eToro
- Compare these top-rated stockbrokers
