
Shares of ImmuPharma PLC (LON: IMM) fell 10% after the company revealed that its US licensing partner Avion was yet to receive a response from the US Food & Drug Administration (FDA) regarding its application for the assessment of Lupuzor’s Phase III trial.
The company clarified that the application was still pending before the FDA in a review queue due to the FDA’s current heavy workload.
Lupuzor is a drug targeted at treating lupus, a systemic autoimmune disease, which usually leads to the bodies immune system attacking its own organs and tissues.
ImmuPharma issued the update given that the 45-day review period usually allocated by the FDA for such applications had passed with no feedback from the regulator.
The pharmaceutical company recently raised £6.5 million through an oversubscribed share placement that triggered a significant drop in its share price since the new shares were priced at a significant discount.
The firm promised to issue an update once it receives a response from the FDA.
Immupharma share price
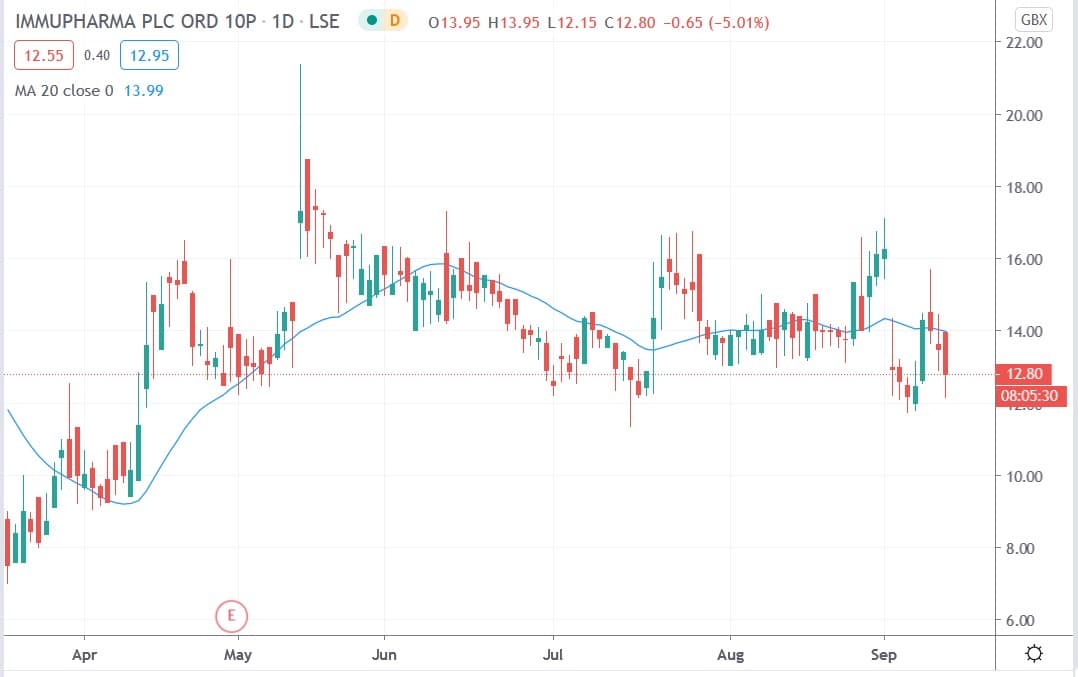
Immupharma shares today fell 10% to trade at 12.15p having ended Thursday’s session trading at 13.50p.
People who read this also read:
- Here are our latest trending stories
- Trade UK shares with the highly-rated IG UK
- Here are the 7 best shares to buy in 2020




