
Shares of Zosano Pharma Corp (NASDAQ: ZSAN) today fell 61.7%% after the company received a discipline review letter (RSL) from the US Food & Drug Administration (FDA) in connection with the Qtrypta™ (zolmitriptan transdermal microneedle system) 505(b)(2) New Drug Application (NDA).
The FDA usually issues a DRL when it identifies deficiencies with a specific review discipline when assessing an NDA. The DRL identified two concerns within the clinical pharmacology section of the NDA.
However, the DRL reflects preliminary comments from the FDA and is not its final decision regarding the NDA, but it indicates that the drub may not be approved by October 20, 2020, which is the Prescription Drug User Fee Act goal date.
“We are disappointed in this notification and are in the process of evaluating and addressing FDA’s comments,” said Steven Lo, president and chief executive of Zosano. “We believe Qtrypta represents an attractive therapeutic alternative for patients suffering from migraines and look forward to working with the FDA through the NDA review process.”
Zosano share price
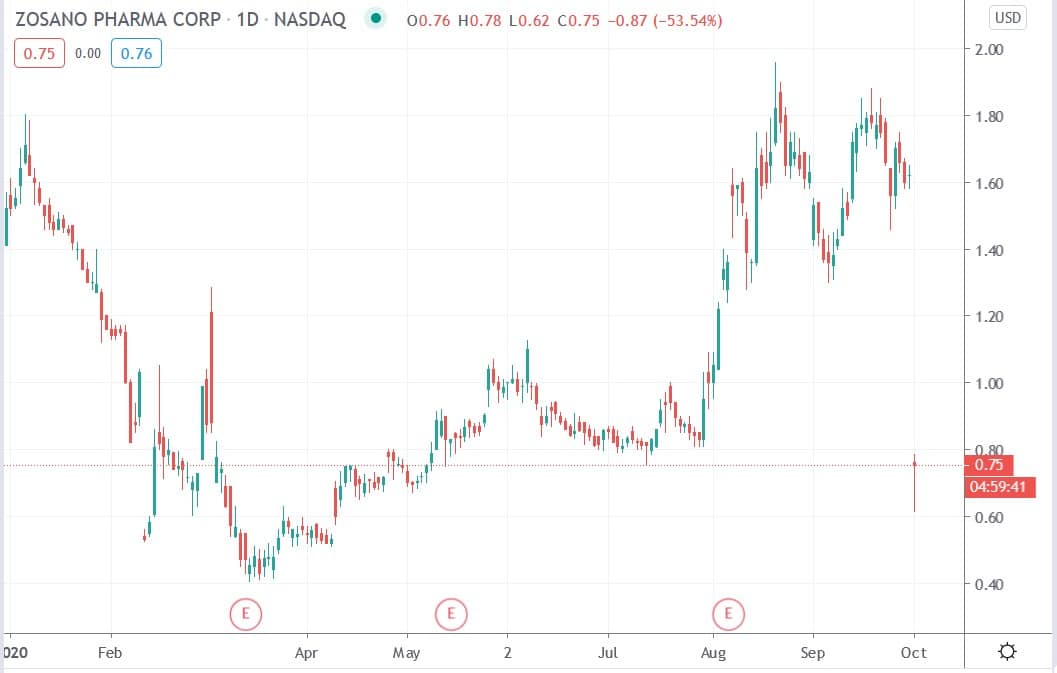
Zosano shares today fell 61.7% to trade at $0.62 having ended Wednesday’s session trading at $1.62.
People who read this also read:
- Here are our latest trending stories
- Trade shares with the highly-rated IG
- Here are the 7 best shares to buy in 2020
