Shares of BioNTech SE (NASDAQ: BNTX) gained nearly 10% Friday on news that a request for Emergency Use Authorization (EUA) will be submitted
Pfizer and BioNTech applied for the EUA with an aim to have it approved in early December. In this case, the first vaccines could eventually be deployed to high-risk groups by the middle to end of December.
“Filing in the U.S. represents a critical milestone in our journey to deliver a COVID-19 vaccine to the world and we now have a more complete picture of both the efficacy and safety profile of our vaccine, giving us confidence in its potential,” said Dr. Albert Bourla, Pfizer Chairman and CEO.
“We look forward to the upcoming Vaccines and Related Biological Products Advisory Committee discussion and continue to work closely with the FDA and regulatory authorities worldwide to secure authorization of our vaccine candidate as quickly as possible.”
As for Europe, two companies plan to seek conditional approval in Europe in the second half of December. Two companies said they will be ready to distribute the vaccine within hours after authorization.
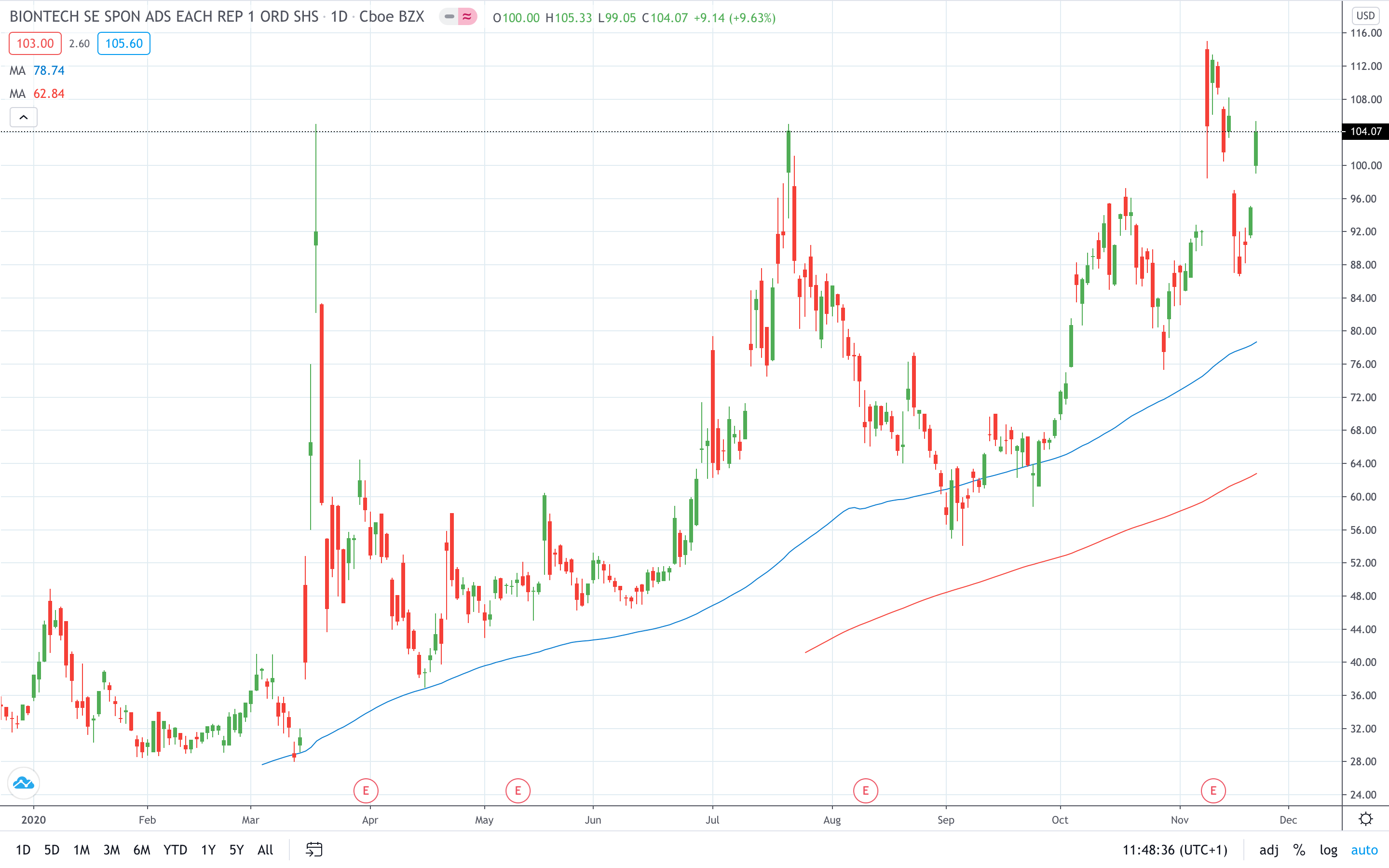
BioNTech share price soared nearly 10% to $104.07.
PEOPLE WHO READ THIS ALSO VIEWED:
- SHARES OF DISNEY GAIN OVER 8% AS DISNEY+ EXCELS
- Learn more on how to open a demo account
- Learn more about Forex Pivot Points
