Key points:
- Sareum shares fell 8.71% despite progressing its drug candidates.
- Investors ignored the multiple positive clinical highlights in the report.
- The decline seems like a natural pullback following the stock’s recent rally.
The Sareum Holdings Plc (LON: SAR) share price fell 8.71% despite revealing that it had progressed one of its drug candidates to the clinical trial, marking a significant milestone for the firm.
Today, the biotech company released its half-year financial results highlighting the progress made in advancing its SDC-1801 treatment, a potential treatment for autoimmune diseases for the acute respiratory symptoms of Covid-19.
Also, Read Five Best Healthcare Stocks in Singapore.
Sareum confirmed that it had completed the final toxicology and safety studies required to apply for an exploratory Clinical Trial Authorisation (“CTA”), with the final report due later in Q1 2022.
The preliminary results from the above studies are aligned with Sareum’s plans to start the first-in-human Phase 1a clinical trial conducted on healthy volunteers while also helping the firm select the initial dosages for the drug.
Today’s decline seems like a natural pullback following the four-day rally in Sareum shares.
Sareum expects to file the CTA for SDC-1801 in mid-2022 and is currently synthesising the SDC-1801 drug substance under GMP conditions to create an oral capsule, with the process being almost complete.
Sareum was also progressing its SDC-1802 cancer immunotherapy drug and was granted a new US patent for the drug in September 2021. The firm is currently conducting translational studies on SDC-1802 to determine the optimal cancer application before completing the toxicology and manufacturing studies.
The company also clarified that Sierra Oncology, which holds the SRA737 selective Chk1 inhibitor patent, is designing the first human clinical trials of several drug candidates, including SRA737.
Sareum will receive $2 million after the first patient dosing in any clinical trial that includes SRA737 under the revised $290 million licensing deal with Sierra Oncology Inc.
The company noted that it had £5.6 million cash at hand on 31 December 2021, having raised £3.9 million in H2 2022.
Dr Tim Mitchell, Sareum’s CEO, commented: “We continue to advance SDC-1801 towards its first clinical trial as a potential new treatment for autoimmune diseases including the acute respiratory symptoms of Covid-19. The preliminary findings from the safety and toxicology studies completed in late 2021 were highly encouraging and gave us confidence in the promising safety profile of SDC-1801. We look forward to receiving the final report in the coming weeks and progressing our plan to begin the first clinical trial with SDC-1801 during H2 2022….”
Adding:
“Furthermore, we are also very encouraged that Sierra Oncology is considering several clinical combination studies with SRA737 and that these may begin in the first half of 2022. We look forward to further updates on the clinical development of this candidate as the programme progresses.”
Sareum shares look attractive at current prices and given the looming 50:1 reverse stock split scheduled for 28 February 2022. Therefore, I would buy the shares.
*This is not investment advice. Always do your due diligence before making investment decisions.
Sareum share price.
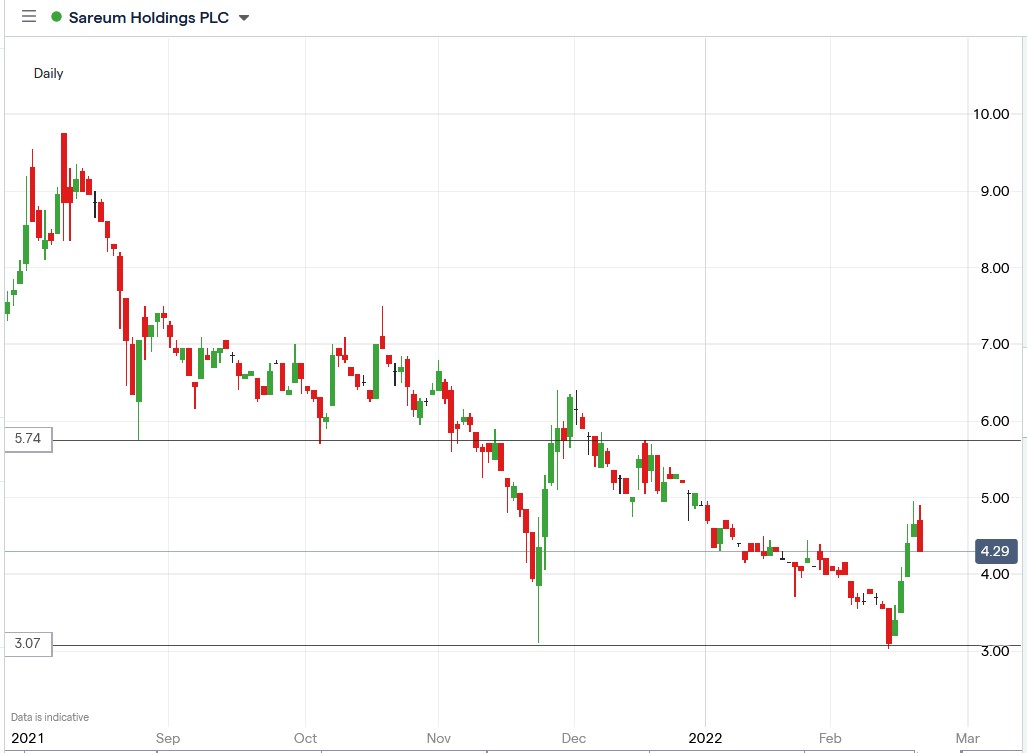
Sareum shares fell 8.71% to trade at 4.29, falling from Friday’s closing price of 4.65p.
